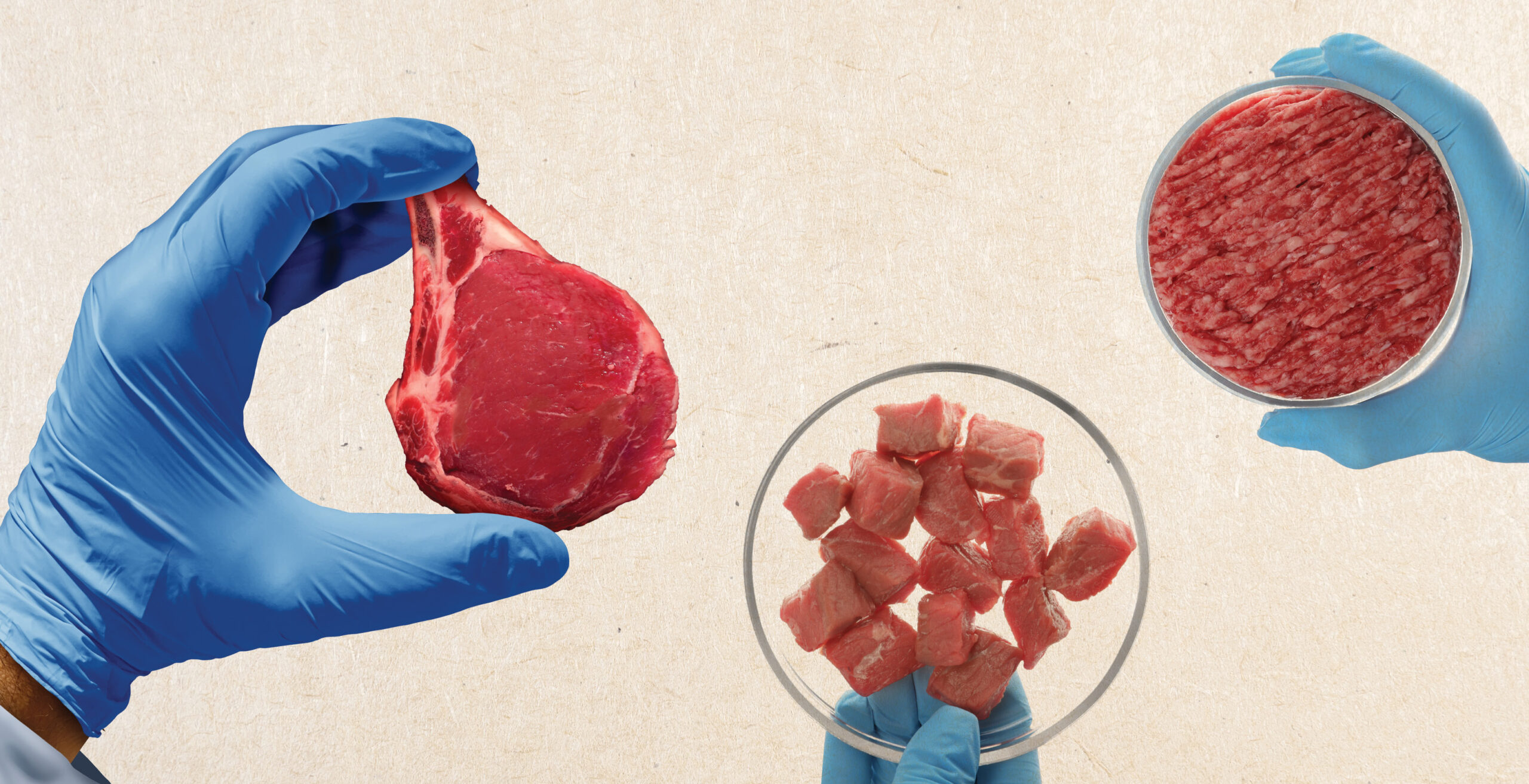
The first cultured beef burger patty was created by Mark Post at Maastricht University in 2013. It was made from over 20,000 thin strands of muscle tissue and cost over $300,000 to produce. The burger was tested on live television in London on August 5, 2013, and was cooked by chef Richard McGeown and tasted by critics Hanni Rützler and Josh Schonwald.
Rützler commented that the consistency was perfect and that it was close to meat, adding that she would have taken the product for meat rather than a soy copy even in a blind trial.
Growth of the Lab Grown Meat Industry
The lab-grown meat industry has seen tremendous growth in recent years, with multiple companies and research institutions working on developing this technology. Some key milestones in this process have been:
In 2020, Singapore became the first country to approve the sale of lab-grown meat, with Eat Just’s cultured chicken product hitting the market.
In 2023, U.S regulators approved the sale of cultured meat to consumers for the first time.
In 2023, Israeli startup SuperMeat obtained kosher certification for their lab-grown chicken products.
Also in 2023, the Chief Rabbi of Israel issued a controversial ruling that the lab grown meat of Aleph Farms can be considered pareve.
The industry is expected to continue growing, with many companies aiming to launch their products in the next few years. The increasing demand for sustainable and ethical food sources, along with advancements in technology, will likely drive the growth of the lab-grown meat industry.
Lab-Grown Meat: The Science and Process Behind the Revolutionary Food
Lab-grown meat, also known as cultured meat or clean meat, is made by growing animal cells in a controlled environment, rather than raising and slaughtering animals. This innovative technology has been gaining attention for its potential to address the ethical, environmental, and health concerns associated with traditional animal agriculture.
But how exactly is lab-grown meat made?
Cell Selection and Isolation
The first step in producing lab-grown meat is to select the type of animal cells to use.
The most used cells for meat production are myocytes (muscle cells) and fibroblasts (connective tissue cells).
In Aleph Farms’ case, the stem cells were obtained from fertilized embryos rather than from muscle tissue cells. In the case of SuperMeat, the stem cells were likewise obtained from a fertilized egg.
Once the cells are isolated, they are cultured in a nutrient-rich medium that promotes cell growth and division. This medium is typically derived from a combination of natural and synthetic sources. Some common components of cell culture media include:
1. SERUM: A protein-rich component derived from animal blood, often from bovine or porcine sources. Serum provides essential nutrients, vitamins, and growth factors for cell growth.
2. ALBUMIN: A protein derived from animal sources, often bovine or human, which helps maintain cell growth and viability.
3. AMINO ACIDS: The building blocks of proteins, essential for cell growth and differentiation.
4. VITAMINS: Essential nutrients like vitamin B12, vitamin E, and others, which support cell health and metabolism.
5. MINERALS: Essential inorganic nutrients like calcium, potassium, and others, which regulate cellular processes.
6. SUGARS: Carbohydrates like glucose or fructose, which provide energy for cell growth.
7. SALTS: Inorganic compounds like sodium chloride, which maintain osmotic balance and support cell growth.
8. GROWTH FACTORS: Proteins that stimulate cell growth and differentiation, such as FGF and VEGF.
9. ANTIBIOTICS: Added to prevent bacterial contamination and promote cell growth.
The cells are then grown in a controlled environment, such as a bioreactor, where temperature, pH, and oxygen levels can be closely monitored and adjusted as needed. This ensures that the cells are provided with an optimal environment for growth and differentiation. The bioreactor also allows for continuous monitoring and control of the cell growth process, enabling the production of large quantities of high-quality cells for meat production.
Cell Proliferation and Differentiation
As the cells grow and divide, they are induced to differentiate into muscle fibers through a process called myogenesis. This process involves mimicking the natural signals that cells receive in the animal body to promote muscle growth.
Tissue Formation and Maturation
Once the cells have differentiated into muscle fibers, they begin to form into tissue-like structures through a process called tissue formation. This process is enhanced by providing a scaffold or matrix for the cells to attach to and grow on. The scaffold serves as a three-dimensional framework that supports the growth and organization of the muscle fibers, allowing them to develop into a cohesive tissue. The scaffold can be made of natural materials, such as collagen or fibrin, which are identical to the extracellular matrix components found in animal tissue, or synthetic materials, such as polylactic acid or polycaprolactone, that mimic the structure and function of natural extracellular matrix. By providing a scaffold that mimics the natural extracellular matrix, the cells can interact with the scaffold and each other in a way that promotes tissue formation, leading to the growth of robust and organized muscle tissue. This tissue formation process allows for the creation of labgrown meat that closely resembles the structure and texture of traditional meat.
As the tissue grows and develops, it requires an increasing amount of nutrients and oxygen to support its expansion and metabolic activity. To address this, tissue is often perfused with a nutrient-rich medium that provides the necessary nutrients, such as glucose, amino acids, and vitamins, and oxygen to support cellular metabolism. This medium also helps remove waste products, such as lactic acid and carbon dioxide, that can accumulate from cellular activity.
Maturation of the tissue is critical to achieve the desired texture and flavor. This process can take several weeks to months, during which time the tissue is monitored for quality and safety.
Halacha
The advent of lab-grown meat has prompted a thorough examination of its Halachic implications. In this multi-part series, we will explore several complex Halachic questions about the Kosher status of such meat. Here are the questions that will be addressed:
1. Non-Kosher stem cells: If the stem cells originate from a non-kosher animal, does this preclude the resulting meat from being considered kosher?
2. Kosher stem cells: Can the stem cells be harvested from a living kosher animal, or is this a form of eiver min hachai?
3. Kosher Slaughter: Must the animal have undergone kosher slaughter for the stem cells to be considered kosher?
4. Salting: Must the animal have undergone “melicha” (salting to remove the blood), before the stem cells are procured?
5. Blood: Can stem cells be derived from animal blood, given that blood is not considered kosher for consumption?
6. Culture Medium: What are the kashrus requirements for the culture medium used to nourish the stem cells? May serum/albumin, which is often derived from animal sources, be utilized?
7. Scaffolding: What are the kashrus requirements for the scaffolding material used to support the growth of the stem cells?
8. Meat Status: Is cultured meat considered fleishig (meat) or pareve (neutral) for the purposes of Halacha?
9. Cell Origin: Does the location within the animal from which the stem cells are extracted impact the meat/pareve status of the resulting cultured meat?
10. Medium and Scaffolding Impact: Do the kashrus requirements of the culture medium and scaffolding material affect the meat/pareve status of the cultured meat?
11. Ma’aras Ayin: How can we address the concern of ma’aras ayin, which dictates that foods must not be prepared in a way that could be misleading or appear to be something they are not, in the case of lab-grown meat that closely resembles traditional meat?
These questions and more will be explored in this multipart series featured in the next few editions of Kosher Spirit.


 EN
EN  ZH
ZH  KR
KR  BR
BR  ES
ES  IN
IN  IL
IL  JP
JP